Since 1981, America’s Essential Hospitals has advanced policies and programs that promote health and access to health care.
We champion our nearly 400 members and their mission to care for low-income and uninsured patients, teach the next generation of health care professionals, and provide lifesaving services for all.


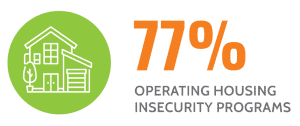
Our hospitals reach outside their walls to create health care access in communities where social and economic disadvantages threaten health and well-being.
Featured Headlines
View all NewsRecent News and Activity
Association Highlights Improper Maximum Fair Price Denials
Institute Brief Details Housing and Homelessness Work at Essential Hospitals
A Legislative Journey: Essential Health System Designation
From the Inside Out: East Alabama Health’s Workforce Development Strategy
Association Highlights Improper Maximum Fair Price Denials
Project Overview: The National Partnership for the Health Care Safety Net
A Legislative Journey: Essential Health System Designation
Preparing for Upcoming Legislative Impacts with Sinai Chicago
Define Essential Health Systems in Federal Law
Report Shows Essential Hospitals’ Impact on Health Care Access, Economic Vitality
Transforming Telehealth for Multilingual Communities
Government Shutdown Inspires Community Table at Bergen New Bridge Medical Center
Featured FAN: Allie Nudelman, MPA
Institute Brief Details Housing and Homelessness Work at Essential Hospitals
Start Your Leadership Journey with the Siegel Fellows Program
From the Inside Out: East Alabama Health’s Workforce Development Strategy
Action for Access and High Quality Health Care
Standing up for Our Nation’s Essential Hospitals
Latest Posts
Innovative Research
Our Essential Hospitals Institute examines and shares practices that elevate health care quality and improve individual and population health.
Institute ResearchLearning, Leadership, Collaboration
America’s Essential Hospitals offers in-person meetings, distance learning events, and collaborative groups for professionals at every level of your organization. Participate in our programming and join hundreds of health care leaders nationwide as they explore key federal and state health care issues, discover innovative and transformative practices, and hone leadership skills.
Upcoming and Recorded Webinars
From the Inside Out: East Alabama Health’s Workforce Development Strategy
East Alabama Health removes barriers to entry and creates sustainable career pathways in health care through local academic partnerships.